Site Master File Eu Guidelines . Based on the explanatory notes for pharmaceutical manufacturers on the preparation of a site. the aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an smf that is useful to the regulatory. the site master file concept has been developed by pic/s and has become a standard expectation of eu authorities. content of a site master fi le. the main objective of the active substance master file (asmf) procedure, formerly known as the european drug master file. explanatory notes on the preparation of a site master file. These notes are intended to provide guidance on the recommended. guideline on the content, management and archiving of the clinical trial master file (paper and/or electronic) keywords. the eu site master file document is available here.
from studylib.net
the main objective of the active substance master file (asmf) procedure, formerly known as the european drug master file. These notes are intended to provide guidance on the recommended. explanatory notes on the preparation of a site master file. Based on the explanatory notes for pharmaceutical manufacturers on the preparation of a site. the eu site master file document is available here. the site master file concept has been developed by pic/s and has become a standard expectation of eu authorities. content of a site master fi le. guideline on the content, management and archiving of the clinical trial master file (paper and/or electronic) keywords. the aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an smf that is useful to the regulatory.
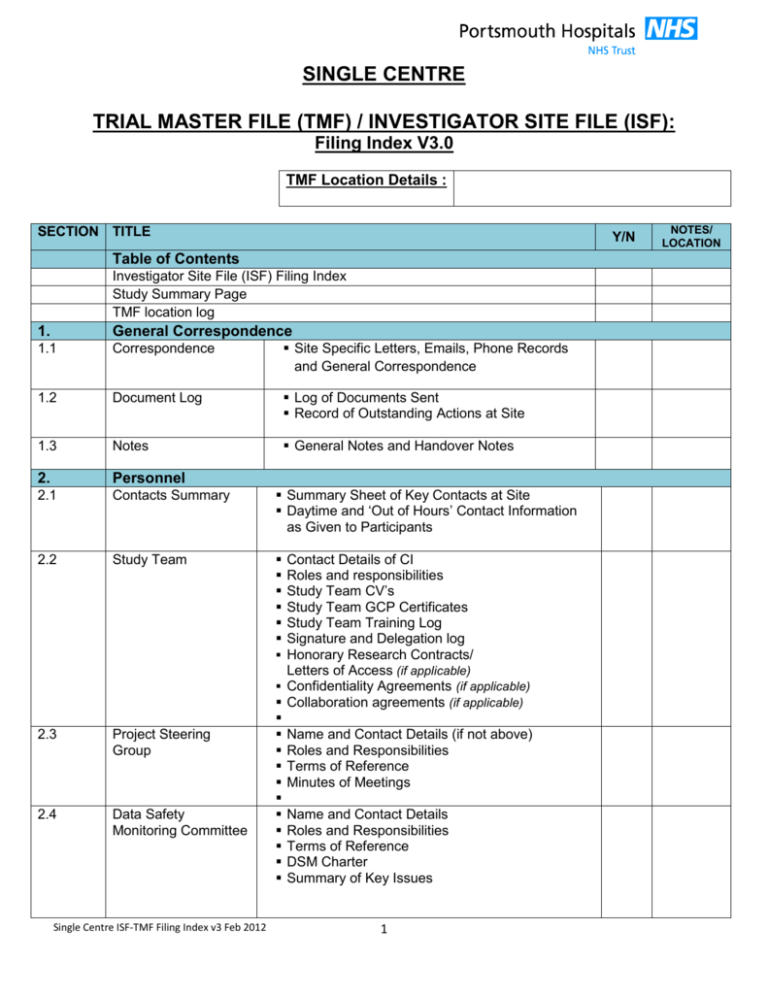
Trial Master File / Investigator Site File Contents
Site Master File Eu Guidelines the eu site master file document is available here. These notes are intended to provide guidance on the recommended. the site master file concept has been developed by pic/s and has become a standard expectation of eu authorities. content of a site master fi le. the aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an smf that is useful to the regulatory. Based on the explanatory notes for pharmaceutical manufacturers on the preparation of a site. guideline on the content, management and archiving of the clinical trial master file (paper and/or electronic) keywords. the eu site master file document is available here. the main objective of the active substance master file (asmf) procedure, formerly known as the european drug master file. explanatory notes on the preparation of a site master file.
From www.telugugmp.com
WHO Guidelines for drafting a site master file/Annex 14 Site Master File Eu Guidelines These notes are intended to provide guidance on the recommended. the site master file concept has been developed by pic/s and has become a standard expectation of eu authorities. the aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an smf that is useful to the regulatory. content of. Site Master File Eu Guidelines.
From www.scribd.com
Site Master File PDF Site Master File Eu Guidelines content of a site master fi le. the aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an smf that is useful to the regulatory. These notes are intended to provide guidance on the recommended. guideline on the content, management and archiving of the clinical trial master file (paper. Site Master File Eu Guidelines.
From www.slideserve.com
PPT Radiopharmaceutical Production PowerPoint Presentation, free Site Master File Eu Guidelines content of a site master fi le. the main objective of the active substance master file (asmf) procedure, formerly known as the european drug master file. These notes are intended to provide guidance on the recommended. the site master file concept has been developed by pic/s and has become a standard expectation of eu authorities. guideline. Site Master File Eu Guidelines.
From www.gbu-presnenskij.ru
Smf Full Form In Pharma Outlet Deals www.gbupresnenskij.ru Site Master File Eu Guidelines the eu site master file document is available here. explanatory notes on the preparation of a site master file. the aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an smf that is useful to the regulatory. Based on the explanatory notes for pharmaceutical manufacturers on the preparation of. Site Master File Eu Guidelines.
From www.slideserve.com
PPT Radiopharmaceutical Production PowerPoint Presentation, free Site Master File Eu Guidelines Based on the explanatory notes for pharmaceutical manufacturers on the preparation of a site. These notes are intended to provide guidance on the recommended. content of a site master fi le. the main objective of the active substance master file (asmf) procedure, formerly known as the european drug master file. explanatory notes on the preparation of a. Site Master File Eu Guidelines.
From www.slideshare.net
Master File Online Resources Updated 2 20 2010 Site Master File Eu Guidelines guideline on the content, management and archiving of the clinical trial master file (paper and/or electronic) keywords. explanatory notes on the preparation of a site master file. These notes are intended to provide guidance on the recommended. the eu site master file document is available here. Based on the explanatory notes for pharmaceutical manufacturers on the preparation. Site Master File Eu Guidelines.
From www.gmp-verlag.de
Site Master File bearbeitbare SOP Download GMPVerlag Peither AG Site Master File Eu Guidelines These notes are intended to provide guidance on the recommended. the eu site master file document is available here. explanatory notes on the preparation of a site master file. the main objective of the active substance master file (asmf) procedure, formerly known as the european drug master file. the aim of these explanatory notes is to. Site Master File Eu Guidelines.
From www.slideshare.net
SITE MASTER FILE Site Master File Eu Guidelines the aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an smf that is useful to the regulatory. These notes are intended to provide guidance on the recommended. explanatory notes on the preparation of a site master file. guideline on the content, management and archiving of the clinical trial. Site Master File Eu Guidelines.
From tech-publish.com
SOP For Site Master File SMF Techpublish Site Master File Eu Guidelines explanatory notes on the preparation of a site master file. the aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an smf that is useful to the regulatory. Based on the explanatory notes for pharmaceutical manufacturers on the preparation of a site. guideline on the content, management and archiving. Site Master File Eu Guidelines.
From www.gmp-verlag.de
Site Master File bearbeitbare SOP Download GMPVerlag Peither AG Site Master File Eu Guidelines guideline on the content, management and archiving of the clinical trial master file (paper and/or electronic) keywords. the aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an smf that is useful to the regulatory. explanatory notes on the preparation of a site master file. the site master. Site Master File Eu Guidelines.
From www.pinterest.com
A Site Master File (SMF) is a document prepared by a manufacturer that Site Master File Eu Guidelines Based on the explanatory notes for pharmaceutical manufacturers on the preparation of a site. These notes are intended to provide guidance on the recommended. the main objective of the active substance master file (asmf) procedure, formerly known as the european drug master file. guideline on the content, management and archiving of the clinical trial master file (paper and/or. Site Master File Eu Guidelines.
From www.scribd.com
site master file Verification And Validation Laboratories Site Master File Eu Guidelines the main objective of the active substance master file (asmf) procedure, formerly known as the european drug master file. the eu site master file document is available here. the aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an smf that is useful to the regulatory. guideline on. Site Master File Eu Guidelines.
From www.yumpu.com
PE 0084 Site Master File PIC/S Site Master File Eu Guidelines the main objective of the active substance master file (asmf) procedure, formerly known as the european drug master file. Based on the explanatory notes for pharmaceutical manufacturers on the preparation of a site. the site master file concept has been developed by pic/s and has become a standard expectation of eu authorities. content of a site master. Site Master File Eu Guidelines.
From www.slideserve.com
PPT THE PREPARATION OF A SITE MASTER FILE SMF PowerPoint Presentation Site Master File Eu Guidelines the main objective of the active substance master file (asmf) procedure, formerly known as the european drug master file. explanatory notes on the preparation of a site master file. the aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an smf that is useful to the regulatory. These notes. Site Master File Eu Guidelines.
From www.pharmasopworld.com
Site Master File in Pharmaceutical Industry Site Master File Eu Guidelines the main objective of the active substance master file (asmf) procedure, formerly known as the european drug master file. content of a site master fi le. the eu site master file document is available here. Based on the explanatory notes for pharmaceutical manufacturers on the preparation of a site. guideline on the content, management and archiving. Site Master File Eu Guidelines.
From basicmedicalkey.com
Site Master File (SMF) and Drug Master File (DMF) Basicmedical Key Site Master File Eu Guidelines guideline on the content, management and archiving of the clinical trial master file (paper and/or electronic) keywords. These notes are intended to provide guidance on the recommended. Based on the explanatory notes for pharmaceutical manufacturers on the preparation of a site. the main objective of the active substance master file (asmf) procedure, formerly known as the european drug. Site Master File Eu Guidelines.
From www.scribd.com
Site Master File Guidance PDF Specification (Technical Standard Site Master File Eu Guidelines Based on the explanatory notes for pharmaceutical manufacturers on the preparation of a site. the main objective of the active substance master file (asmf) procedure, formerly known as the european drug master file. explanatory notes on the preparation of a site master file. the aim of these explanatory notes is to guide the manufacturer of medicinal products. Site Master File Eu Guidelines.
From easymedicaldevice.com
GSPR General Safety And Performance Requirements [EU MDR & IVDR] Site Master File Eu Guidelines Based on the explanatory notes for pharmaceutical manufacturers on the preparation of a site. the eu site master file document is available here. the aim of these explanatory notes is to guide the manufacturer of medicinal products in the preparation of an smf that is useful to the regulatory. content of a site master fi le. Web. Site Master File Eu Guidelines.